The COVID-19 pandemic exposed critical vulnerabilities in the global healthcare supply chain. It demonstrated the need for medical device manufacturers to quickly evaluate various production scenarios such as increasing throughput, executing rapid product changeovers or optimizing operations with a reduced workforce.
It was crucial to adjust approved product designs or production plans in an extremely short turnaround time or to evaluate a device’s performance and safe operation in a previously untested situation. Leveraging simulation and building an executable digital twin (xDT) can speed up innovation processes, resulting in rapid design enhancements, prototyping and validation of medical devices.
This webinar features ways to win the race against time, innovate medical devices quickly and advance product development with the executable digital twin.
An executable digital twin helps answer key challenges brought on by COVID-19
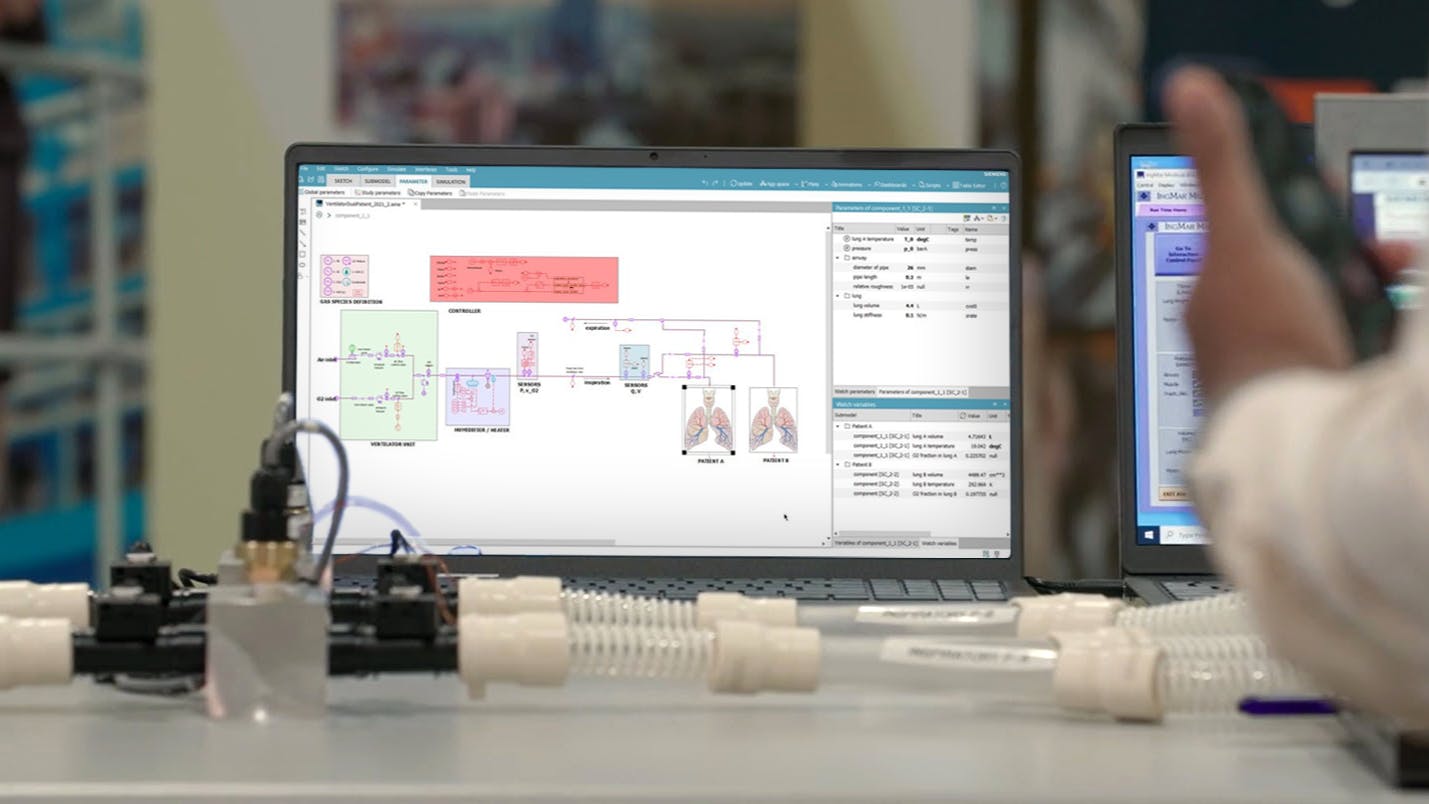
At the height of the pandemic there was a surge in patients suffering from respiratory infections arriving at hospitals. Physicians had to place two patients on a single ventilator due to insufficient inventory, without knowing if it was safe, and address two critical issues:
- Can the ventilator lines be split effectively?
- Can a single ventilator support two patients with similar lung conditions?
In this webinar you’ll learn from a real case and find out how Siemens used Simcenter solutions to answer both questions. For background about the project, you are encouraged to watch this short video prior to watching.
In this seminar you will learn:
- The key benefits of adopting digital solutions to advance development procedures
- About the digital design development and engineering analysis using Simcenter 3D for fluid dynamics and structural analysis, of a disposable assembly, consisting of a splitter and two one-way valves, for use during shared ventilation
- How Simcenter systems simulation was used to model the dual patient-single ventilator system and to assess the ability of the ventilator to maintain two patients in this untested scenario
- How the system model can be used further as the “executable digital twin” to provide real time insights during ventilator operation
- How to build such a system model and create a Functional Mockup (FMU) that can be executed on edge devices for monitoring system status and provide real-time insights for control
- About the value of system simulation in virtually testing the ventilator or any medical device in an unplanned event
Watch the on-demand webinar today!
Conheça os palestrantes

Elena Arvanitis
Project Manager

Mark Carlson
Sr. Principal Technical Consultant - Lifesciences Simulation & AI

Gibin Joe Zachariah
Simcenter Engineering Services Engineer