Shortening the amount of time it takes to get products to market—this is a concern shared by all manufacturers across all industries. In the medical device industry, this objective takes on additional importance, as it means that lifesaving and life-sustaining devices and treatments can get to patients more quickly. In the case of respiratory devices, Computational Fluid Dynamics (CFD) simulation is a critical enabling technology for realizing this objective, as will be discussed in this webinar, with guest speaker Dr. Chris Varga, Senior Engineering Fellow at Vyaire Medical.
CFD simulation is a key technology at every stage of device development
How pervasive is the use of simulation at Vyaire? “I can’t think of any product that is in development today that we’re not using CFD for,” says Dr. Varga. At Vyaire, CFD is used in early stage research, feasibility studies, and design studies performed prior to building prototypes. Concepts for device designs will sometimes start within the simulation tool—the same tool that is also used to resolve issues encountered much later in the device development process, including during manufacturing.
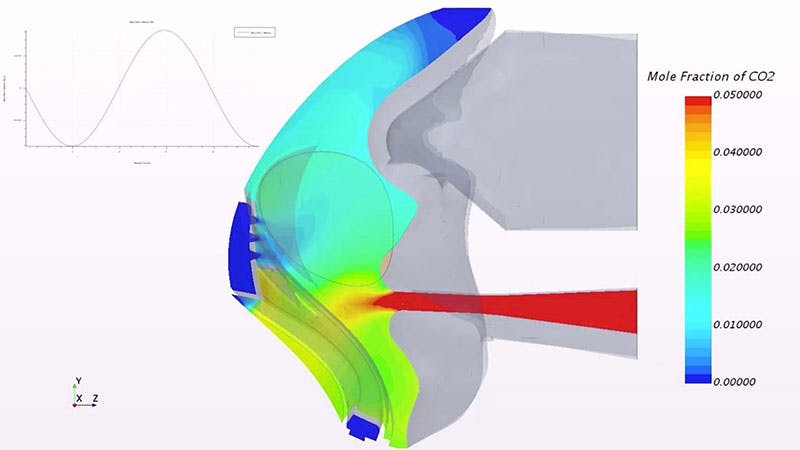
And since patients come in a variety of shapes and sizes and also breathe in a variety of ways (some people are "mouth breathers”, some are "nose breathers"), simulations must account for the fact that each patient has a unique breathing profile that depends on their lung function and structure. The ability to perform detailed simulations of all their use cases, combined with the time and cost savings resulting from a reduced reliance on expensive prototypes represent just two of the ways in which Vyaire’s investment in simulation has paid off.
What you will learn in this webinar:
- How CFD simulation is used in the development of differentiated respiratory and anesthesia care devices, among others, at Vyaire
- How Vyaire incorporates patient-specific data in their simulations, to maximize the effectiveness of their devices across the intended patient populations
- Future applications of simulation in the development of medical devices
Presenters:
Dr. Chris Varga, Senior Engineering Fellow, Vyaire Medical
Javier Garriz, Marketing Manager, Siemens Digital Industries Software