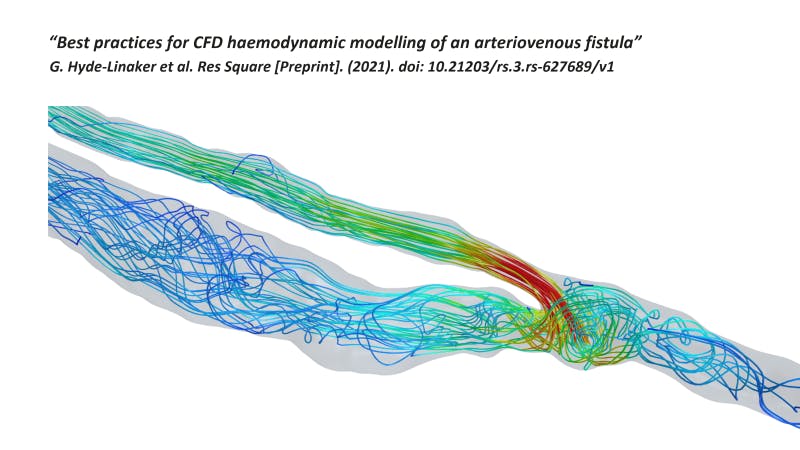
Simulation is long established as a method for generating proof that a medical device meets a requirement. But simulation can be used in other ways as well. Watch the on-demand webinar to learn about best practices for computational fluid dynamics (CFD) haemodynamic modeling and how other organizations have used simulation for diagnosis and intervention planning.
Build a credible model for CFD haemodynamics
Simulation is not useful unless it is credible. There must be trust in the predictive capabilities of a computational model in the specific context of where it is being used. Once that credibility is established, organizations can use simulation all throughout the design lifecycle. From early concept building where costs are relatively low, simulation can help to reduce the number of prototypes needed and therefore the ability to get a product to market sooner.
Achieve detailed analysis of the haemodynamic environment and metrics
The majority of CFD haemodynamic studies use velocity inlet–pressure outlet boundary conditions. The inlets are prescribed to be the velocity or mass flow waveforms. In regard to outlets, there are a few options, including zero-pressure or “bleeding out” outlet, prescribed flow split percentages based on daughter vessel areas, or Windkessel models. Lastly, for walls, non-slip conditions versus fluid-structure interaction methods can be used.
What you will learn from this webinar
- How to implement best practices for CFD haemodynamic modelling in Simcenter STAR-CCM+
- How to compute key haemodynamic metrics within Simcenter STAR-CCM+
- How to automate a repeatable and efficient CFD pre-processing pipeline
- How to couple Simcenter Amesim and Simcenter STAR-CCM+ for zero-dimensional Windkessel models
Meet the speakers

Javier Garriz
Solution Consultant for Medical Devices

George Hyde-Linaker
Department of Biomedical Engineering

Dr. Asimina Kazakidi, FHEA, MIPEM, RSE ΜYAS
Senior Lecturer, Director of MSc/MRes programmes in Biofluid Mechanics